Abstract

CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic ratio. Intact CPX-351 liposomes containing the synergistic ratio are taken up by leukemia cells to a greater extent versus normal bone marrow cells, have a high retention of drug cargo, and have a long plasma half-life. In a phase 3 study in older adults with newly diagnosed, treatment-related AML or AML with myelodysplasia-related changes, CPX-351 demonstrated significantly improved overall survival and response rates compared with conventional 7+3 chemotherapy and a safety profile consistent with that of 7+3. Cumulative lifetime exposure to daunorubicin is associated with risks of cardiotoxicity. Therefore, it was of interest to determine if the superior efficacy observed with CPX-351 versus 7+3 was achieved with lower cumulative doses of cytarabine and daunorubicin relative to 7+3. An exposure-response analysis was conducted to document the relationships of cumulative doses of cytarabine and daunorubicin with efficacy outcomes for CPX-351 versus 7+3 therapy.
Efficacy and cumulative exposure data were obtained from the phase 3 study CLTR0310-301 (NCT01696084), in which patients were randomized 1:1 to receive CPX-351 or 7+3 induction therapy. Cumulative dose corresponded to the total amount of drug (mg of cytarabine or daunorubicin) administered to an individual patient during the entire time that patient remained on the study. The clinical endpoints for efficacy were overall survival, complete remission (CR), and CR + CR with incomplete hematologic recovery (CR+CRi).
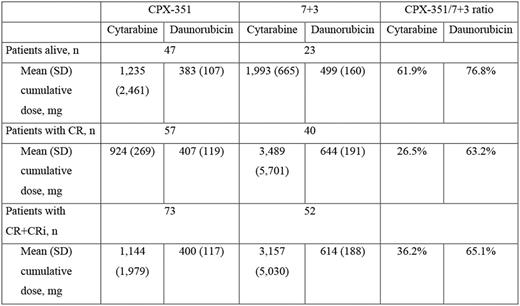
A total of 309 patients were enrolled in the study; the exposure-response analysis for cumulative dose included 153 patients treated with CPX-351 and 149 patients treated with 7+3. Baseline characteristics were generally similar between the CPX-351 and 7+3 cohorts. In the overall study population, median duration of the treatment was 62 days in the CPX-351 arm and 41 days in the 7+3 arm. The most common reason for discontinuation among patients included in this analysis was lack of efficacy, but the incidence was lower for the CPX-351 cohort (34.6%) than for the 7+3 cohort (49.0%). In the exposure-response analysis for overall survival, patients treated with CPX-351 who were alive at the data cut-off time for the primary endpoint analysis (n = 47) tended to have received less cytarabine and daunorubicin than those treated with 7+3 (n = 23). The mean cumulative doses of cytarabine and daunorubicin for patients alive in the CPX-351 cohort were 61.9% and 76.8%, respectively, of cumulative doses for patients alive in the 7+3 cohort (Table). Among responders classified as achieving a CR or CR+CRi, those treated with CPX-351 tended to receive less cytarabine and daunorubicin than those treated with 7+3 chemotherapy. The mean cumulative doses of cytarabine and daunorubicin for patients who achieved a CR in the CPX-351 cohort were 26.5% and 63.2%, respectively, of cumulative doses for patient with CR in the 7+3 cohort, and the mean cumulative doses for patients who achieved a CR+CRi in the CPX-351 cohort were 36.2% and 65.1%, respectively, of those with CR+CRi in the 7+3 cohort (Table). For non-responders or deceased patients, the mean cumulative doses of cytarabine and daunorubicin similarly tended to be lower in patients who received CPX-351 than in those who received 7+3 therapy. The finding that a lower cumulative dose of cytarabine and daunorubicin in CPX-351 relative to standard 7+3 therapy led to improved efficacy and increased on-target myelosuppression supports the observation in animal models that CPX-351 is taken up in the bone marrow by leukemia cells to a greater extent than normal cells.
Taken together, the results of this exposure-response analysis show that the mean cumulative doses of cytarabine and daunorubicin associated with an overall survival benefit were lower with CPX-351 than with 7+3, as were the mean cumulative doses associated with achieving a CR or CR+CRi. Thus, compared with 7+3, CPX-351 was associated with improved efficacy at a reduced total drug burden in this population of older adults with newly diagnosed, treatment-related AML or AML with myelodysplasia-related changes.
Banerjee: Celator/Jazz: Employment, Equity Ownership. Wang: Celator/Jazz: Employment, Equity Ownership. Wang: Celator/Jazz: Consultancy. Gibbons: Celator/Jazz: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal